A2. Amino Acid Stereochemistry
- Page ID
- 4688
The amino acids are all chiral, with the exception of glycine, whose side chain is H. As with lipids, biochemists use the L and D nomenclature. All naturally occurring proteins from all living organisms consist of L amino acids. The absolute stereochemistry is related to L-glyceraldehyde, as was the case for triacylglycerides and phospholipids. Most naturally occurring chiral amino acids are S, with the exception of cysteine. As the diagram below shows, the absolute configuration of the amino acids can be shown with the H pointed to the rear, the COOH groups pointing out to the left, the R group to the right, and the NH3 group upwards. You can remember this with the mnemonic CORN.
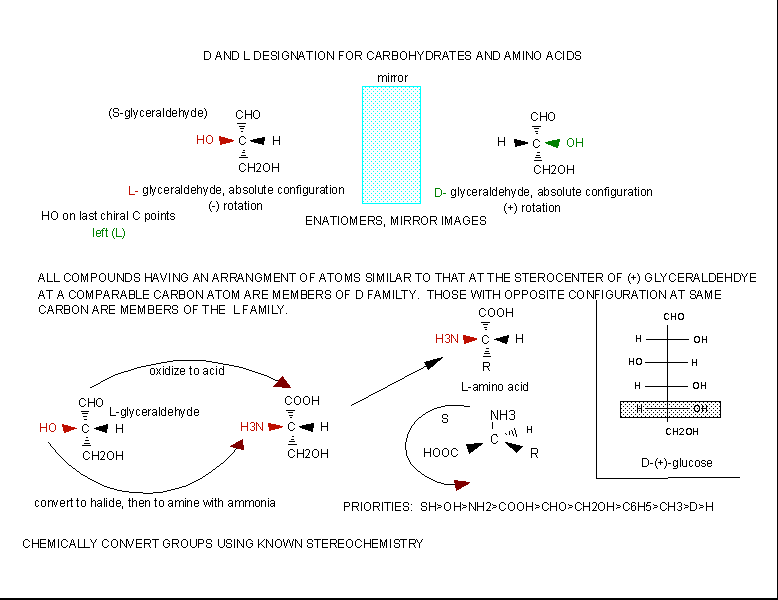
Figure: Stereochemistry of Amino Acids.
Why does Biochemistry still use D and L for sugars and amino acids? This explanation (taken from the link below) seems reasonable.
"In addition, however, chemists often need to define a configuration unambiguously in the absence of any reference compound, and for this purpose the alternative (R,S) system is ideal, as it uses priority rules to specify configurations. These rules sometimes lead to absurd results when they are applied to biochemical molecules. For example, as we have seen, all of the common amino acids are L, because they all have exactly the same structure, including the position of the R group if we just write the R group as R. However, they do not all have the same configuration in the (R,S) system: L-cysteine is also (R)-cysteine, but all the other L-amino acids are (S), but this just reflects the human decision to give a sulphur atom higher priority than an oxygen atom, and does not reflect a real difference in configuration. Worse problems can sometimes arise in substitution reactions: sometimes inversion of configuration can result in no change in the (R) or (S) prefix; and sometimes retention of configuration can result in a change of prefix.
It follows that it is not just conservatism or failure to understand the (R,S) system that causes biochemists to continue with D and L: it is just that the DL system fulfils their needs much better. As mentioned, chemists also use D and L when they are appropriate to their needs. The explanation
given above of why the (R,S) system is little used in biochemistry is thus almost the exact opposite of reality. This system is actually the only practical way of unambiguously representing the stereochemistry of complicated molecules with several asymmetric centres, but it is inconvenient with regular series of molecules like amino acids and simple sugars. "
If I told you to draw the correct stereochemistry of a molecule with 1 chiral C (S isomer for example) and I gave you the substituents, you could do so easily following the R, S priority rules. However, how would you draw the correct isomer for the L isomer of the amino acid alanine? You couldn't do it without prior knowledge of the absolute configuration of the related molecule, L glyceraldehyde, or unless you remembered the mnemonic CORN. This disadvantage, however, is more than made up for by the fact that different L amino acids with the same absolute stereochemistry, might be labeled R or S , which makes this nomenclature unappealing to biochemists.


