C5: Membrane Permeability
- Page ID
- 4682
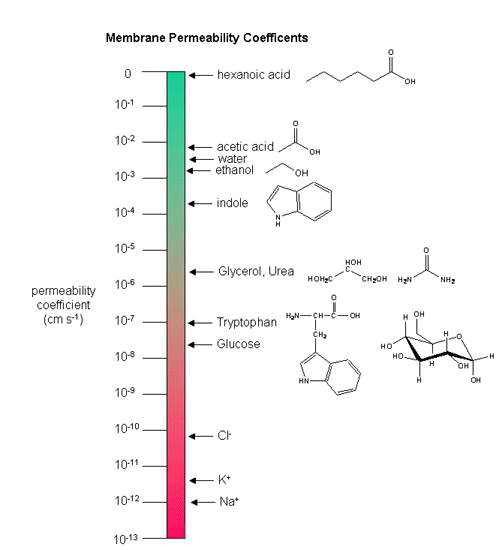
Another indicator of membrane dynamics is the measured permeability coefficient of ions and molecules across the bilayer. (We will discuss these coefficients in the chapter on Passive and Facilitated Diffusion in the Transport and Kinetics Unit.) Permeabilities are correlated to the partition coefficient of the molecule or ion in organic solvents. If the molecule readily dissolves in a nonpolar solvent, it is more likely to pass through the hydrophobic barrier of the membrane. Obviously the size of the molecule would play a part as well. Hence charged ions like Na+ and K+ have low permeabilities. Cl- has a higher permeability, since the charge density on the larger Cl- anion is smaller. Water has a surprisingly high permeability although it is polar. It is small and can enter down deep into the head group region through sequential H-bonding which must assist its transfer across the membrane.
Figure: Permeability of liposome bilayers
Many different probes have been developed to study membrane structure and dynamics. Many of them are fluorescent. The diagram below, taken from the Molecular Probe's catalog, gives examples.


